
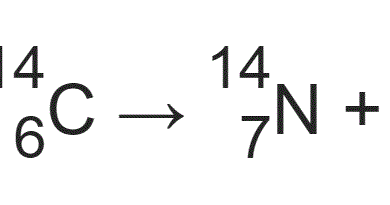
It was a brilliant undertaking for which Libby was awarded the Nobel prize for chemistry in 1960, though he was lucky in one sense. His results perfectly matched the known dates of the items he had scanned.
#Carbon 14 decay constant series#
Libby then provided final proof of his dating technology by measuring the radioactivity – and, by inference, the age – of a series of organic samples of known antiquity: wood from the Egyptian funeral ship of Sesostris III, linen that had wrapped a Dead Sea scroll and a bread roll that had been “cooked” in the volcanic eruption that buried Pompeii. As Marra says: “Human waste from sewer lines sent science onward.” By contrast, methane newly excreted by humans was relatively rich in the isotope.
It should be rich in carbon-14, having just been produced by humans, Libby reasoned.Īnd that is exactly what he found. The second came from the city of Baltimore sewerage system and was extracted from human excrement. One sample was extracted from natural gas, a fossil fuel whose carbon-14 should have decayed long ago. Then he turned to the gas methane, which contains carbon, to provide final validation of his technique, comparing samples from two very different sources. Libby solved the problem by carefully shielding his detectors and developing ways to tune out any radiation that made it through to the walls of his device.
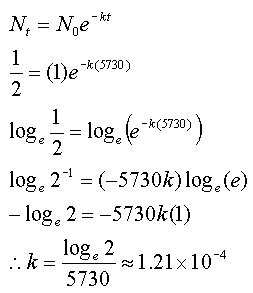
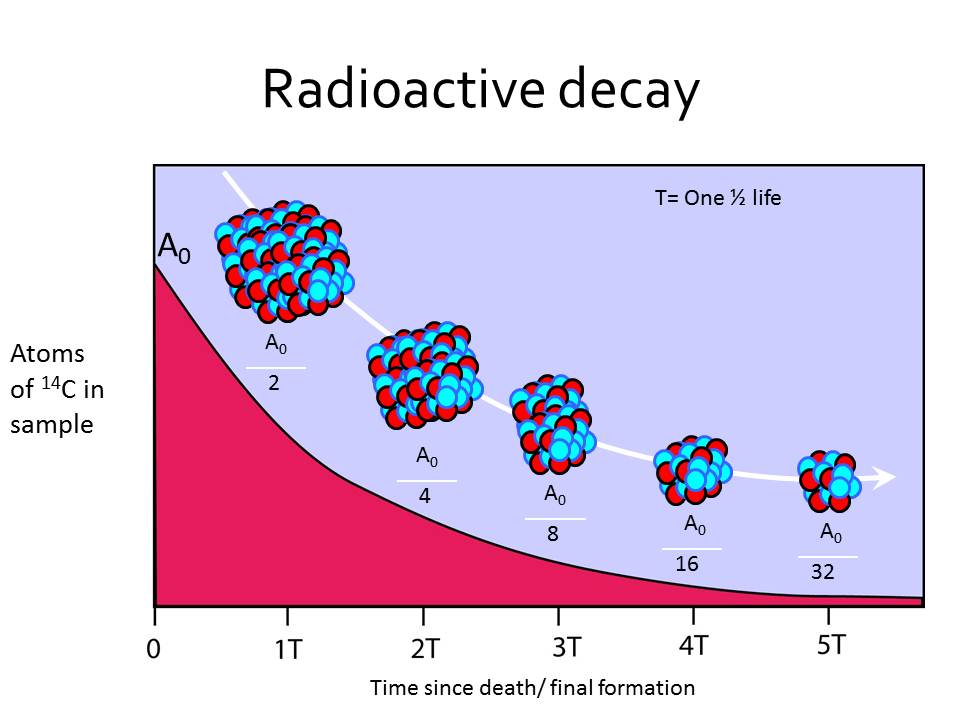
How could researchers separate carbon-14’s weak signal from this overwhelming background noise? We have gained substantial understanding of the natural world because of carbon-14 John Marra By contrast, natural background radiation – from thorium and uranium in rocks and other sources – is much, much higher. Carbon-14 exists in only very low levels in the tissue of recently deceased animals and plants: about one in a trillion of their carbon atoms are carbon-14. The sciences of archaeology and palaeontology were about to be revolutionised.Ī major problem had to be overcome, however. So, by measuring the radioactivity of a sample taken from the organism, its carbon-14 content could be estimated and the date of its time of death could be measured. A chemist who had worked on the Manhattan Project to build the first atom bomb, Libby realised that when an organism dies, it will stop absorbing carbon, including carbon-14, and its existing store of the latter will slowly decay. “Every living thing on Earth thus becomes radioactive, albeit slightly,” says Marra.Īnd it dawned on Willard Libby of Chicago University that the radioactivity generated by carbon-14 could be exploited to tremendous advantage. In turn, these atoms combine with oxygen to create radioactive carbon dioxide that is absorbed by plants, which are then eaten by animals. These neutrons strike atoms of nitrogen, the main component of Earth’s atmosphere, and transform some into atoms of carbon-14. Cosmic rays batter the upper atmosphere and send cascades of neutrons through the air, they calculated. And since the discovery of a long-lived radioisotope of carbon, we have an amazing tool to delve into almost every aspect of existence on Earth – and perhaps the universe.”Īs Marra reveals in this remarkable history of carbon-14, scientists quickly realised the isotope must affect living beings today.
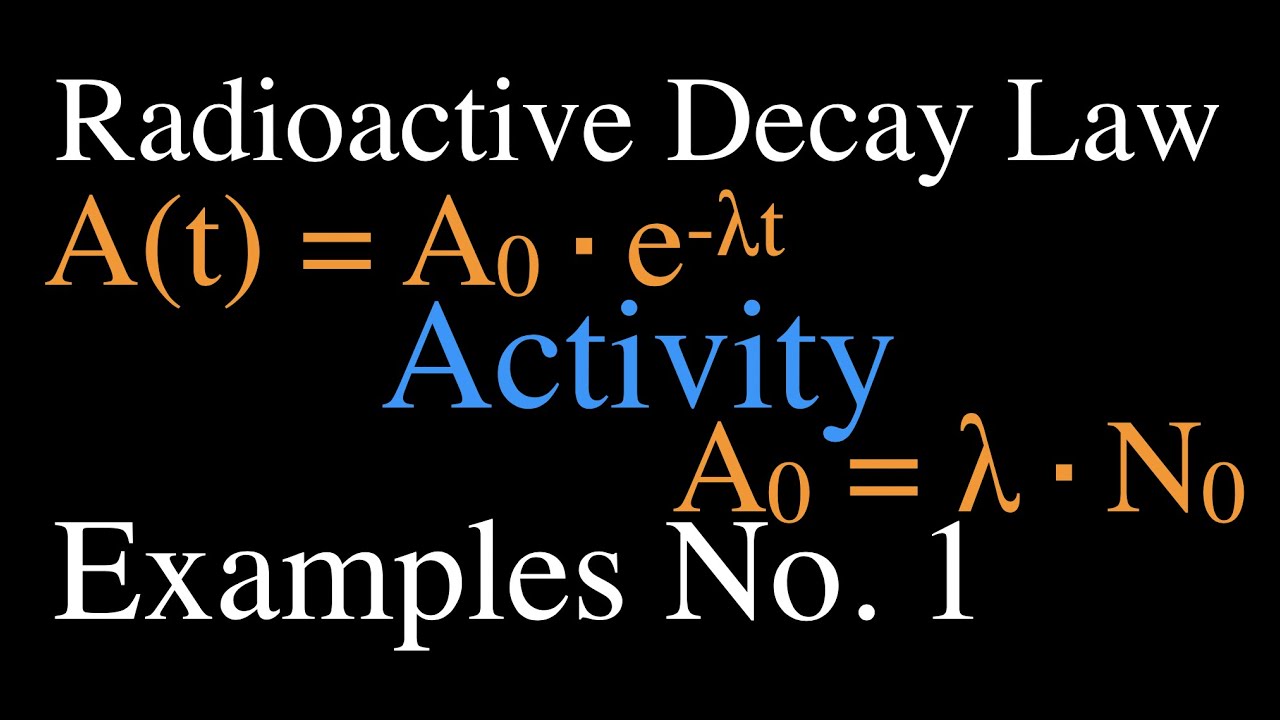
It is fundamental also to how we live, how the Earth is habitable – pretty much everything. “Carbon is what we are made of,” says Marra, who is professor of earth and environmental sciences at Brooklyn College, New York.


 0 kommentar(er)
0 kommentar(er)
